In an era where the pharmaceutical industry grapples with unprecedented data volumes—estimated at 400 exabytes generated globally each day, equivalent to 18,000 trillion books—the integration of artificial intelligence (AI) into drug manufacturing is no longer a futuristic dream but a pressing necessity. The Parenteral Drug Association (PDA) Regulatory Conference 2025, held September 8–10 in Washington, DC, brought together industry leaders, regulators, and innovators to dissect this transformation. Titled “Data Governance and AI’s Impact on Drug Manufacturing,” the discussions underscored how robust data strategies are fueling AI-driven efficiencies, compliance, and patient safety.
This blog post dives deep into the conference highlights, backed by hard data, expert quotes, and actionable insights. From skyrocketing market projections to real-world case studies, we’ll explore how AI is reshaping drug production. Whether you’re a pharma executive, quality assurance specialist, or tech enthusiast, buckle up—this is your comprehensive guide to the AI revolution in manufacturing.

The PDA 2025 Conference: A Hub for Pharma Innovation
The PDA Regulatory Conference 2025 wasn’t just another gathering; it was a clarion call for digital maturity in biopharma. With sessions spanning AI deployment, GxP compliance, and supply chain oversight, the event highlighted the industry’s shift toward data-centric operations. Attendees polled during sessions revealed that while few companies have fully approved AI strategies for quality control (QC), most have established governance policies— a sign of cautious optimism.
Key agenda items included:
- Revolutionizing Process Design with GenAI: The Kindeva Approach – Showcasing generative AI for automating SOPs and risk assessments in fill-finish facilities.
- Operational Efficiency in Viral Vector Manufacturing Using AI – Focusing on machine learning to cut costs in GMP plasmid production.
- Factory of the Future – Autonomous Manufacturing – CEO Casper Hansen of Technicon A/S discussed robotics reducing contamination risks by up to 50% in high-stakes environments.
Digitalization maturity scores have climbed to 3.5 out of 5 (from 2.6 in 2019), per recent surveys, but challenges like cybersecurity and validation persist. The conference’s collaborative spirit, including roundtables on AI deployment, emphasized cross-functional training and regulator engagement to bridge these gaps.
The Data Deluge: Why Pharma is Drowning in Information
Pharma generates massive unstructured data daily—think batch records, sensor readings, and genomic sequences. Globally, 400 exabytes of data are produced each day, with much of it unstructured and ripe for AI analysis. In drug manufacturing alone, integrating data from programmable logic controllers (PLCs) and batch systems can reveal hidden inefficiencies, like variable raw material impacts on yields.
Here’s a snapshot of pharma’s data explosion:
| Data Metric | 2024 Estimate | 2025 Projection | Growth Rate |
|---|---|---|---|
| Global Pharma Data Volume | 2.3 zettabytes | 3.1 zettabytes | 35% YoY |
| Unstructured Data Share | 80% | 85% | +5% |
| Daily Sensor Data in Manufacturing | 1 petabyte/site | 1.5 petabytes/site | 50% increase |
Sources: Industry surveys and AI market reports.
Toni Manzano, PhD, co-founder of Aizon, captured the essence: “This data deluge, combined with the widespread availability of computing power and data storage, is fueling an artificial intelligence (AI) renaissance that promises to redefine drug discovery, development, and manufacturing.” Without governance, this deluge becomes a liability—over 25% of FDA warning letters since 2019 cite data accuracy issues.
AI’s Transformative Role in Drug Manufacturing
AI isn’t just hype; it’s delivering measurable gains. At PDA 2025, sessions showcased AI predicting batch success for advanced therapy medicinal products (ATMPs) an hour in advance, in partnership with the European Medicines Agency (EMA). This predictive power minimizes waste and accelerates release, crucial for time-sensitive therapies like CAR-T cells.
Key Use Cases from the Conference
- Process Optimization: AI integrates batch records with PLC data to adjust pH in plasma fractionation, boosting yields by 15-20% without trial-and-error.
- Quality Control Automation: AbbVie’s AI-driven CMO scorecard automates data refreshes, slashing manual processing time by 90% and enhancing supplier transparency.
- Supply Chain Oversight: Generative AI codes complaints in seconds (vs. minutes), enabling real-time escalation and CMO collaboration.
In viral vector manufacturing, AI optimizes cell lines and plasmids, reducing costs by 30% through predictive analytics. Broader impacts include digital twins for simulating production lines, cutting downtime by 25%.
Market Data: AI’s Economic Boom
The AI in pharma market is exploding:
| Year | Market Size (USD Billion) | CAGR | Key Driver |
|---|---|---|---|
| 2024 | 3.24 | – | GenAI Adoption |
| 2025 | 5.12 | 58% | Regulatory Alignment |
| 2033 | 65.83 | 45% | Manufacturing Efficiency |
| Annual Value Creation | 350-410 | – | By 2025 |
Data from Roots Analysis and McKinsey reports.
By 2025, 75% of pharma companies will prioritize generative AI, potentially unlocking $250 billion in efficiency gains.

Global AI in Drug Manufacturing Market Size and Trends 2040
Data Governance: The Unsung Hero of AI Success
Data preparation devours 80% of AI project time, making governance non-negotiable. PDA 2025 stressed FAIR principles (Findable, Accessible, Interoperable, Reusable) to turn raw data into AI fuel. Vinny Browning of Amgen advocated embedding AI in quality management systems (QMS), with clear gating for GMP relevance.
Frameworks and Best Practices
- Risk Management: Structured registries for AI risks, including model drift and cybersecurity.
- Validation: Lifecycle-focused approaches for evolving AI, per FDA’s January 2025 draft guidance.
- Training: Cross-functional programs to ensure staff can explain AI during audits.
Browning noted: “Data integrity is paramount, and inconsistencies in data naming or definitions across systems… can undermine AI effectiveness.” In roundtables, participants identified validation uncertainty as the top hurdle, calling for AI-specific SOPs.
Challenges: From Validation to Ethical AI
Despite the promise, PDA 2025 didn’t shy from pitfalls. High startup costs, workforce readiness, and explainability loom large. Generative AI isn’t yet QC-ready due to autonomy risks—human oversight remains key.
| Challenge | Impact | Mitigation Strategy |
|---|---|---|
| Data Inconsistencies | 25% FDA Warnings | FAIR Compliance |
| Cybersecurity | Supply Chain Breaches | Redundant Systems |
| Validation Gaps | Delayed Deployments | Lifecycle Frameworks |
| Training Needs | Low Adoption | Digital Readiness Programs |
Derived from conference roundtables.
Regulatory convergence—FDA, EMA Annex 11 revisions—is underway, but 2025 will test compliance in areas like patient privacy.
Real-World Examples: AI in Action at PDA 2025
- Amgen’s Digitization: AI aggregates deviations for annual reviews, saving “numerous hours” of manual work.
- Kindeva’s GenAI: Automates 100+ micro-processes, from risk assessment to training.
- Insilico Medicine: CEO Alex Zhavoronkov predicts fully AI-designed drugs by 2030, with manufacturing optimizations slashing timelines by 70%.
These cases illustrate AI’s shift from job aid to autonomous ops, always with human verification.
The Road Ahead: Predictions for 2025 and Beyond
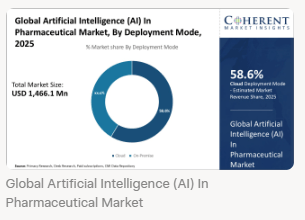
By 2025, AI could halve drug development costs ($2B average) via predictive manufacturing. Expect more EMA-FDA pilots for ATMPs and cloud deployments dominating (58.6% market share).
Manzano reminded: “Everyone in this room… we are working for patients, so we have to [always] have in mind that everything we do is because there is a patient waiting.” With 90% of AI models now industry-sourced, pharma’s innovation edge sharpens.
Conclusion: Embrace AI with Governance at the Helm
PDA 2025 painted a vivid picture: AI, powered by ironclad data governance, is set to revolutionize drug manufacturing. From 400 exabytes of daily data to $65B markets by 2033, the numbers don’t lie. But success hinges on addressing challenges head-on—through FAIR principles, robust QMS, and patient-first mindsets.
As we close 2025, pharma leaders must invest in training and pilots. The patient waiting at the end of the line deserves no less. What’s your take on AI’s role? Share in the comments below!
Sources and further reading: PDA.org, BioPharm International, FDA Guidance.
Leave a comment