
In 2025, the convergence of artificial intelligence (AI) and synthetic biology (SynBio) is not just a scientific milestone—it’s a paradigm shift. Synthetic biology, the discipline that reprograms the code of life—DNA—to build custom organisms, has long promised to revolutionize medicine, agriculture, energy, and beyond. Yet its complexity, rooted in biology’s unpredictable molecular dance, has often slowed progress. Enter AI, wielding computational prowess to design, predict, and automate biological systems with unprecedented precision. This fusion is ushering in a “biosingularity,” where life itself becomes as programmable as software.
This comprehensive blog post explores the scientific foundations, transformative breakthroughs, economic impacts, ethical challenges, and future horizons of AI-driven SynBio. Through peer-reviewed insights, real-world examples, and data-driven visualizations, we’ll unpack how this synergy is rewriting life’s possibilities—and what it means for humanity.
The Foundations of Synthetic Biology: Life as a Designable System
Synthetic biology merges biology with engineering, treating cells as programmable platforms. Unlike traditional genetic engineering, which tweaks existing genes, SynBio designs entirely new biological systems or radically re-engineers natural ones. Its core premise builds on molecular biology’s central dogma: DNA encodes instructions, transcribed to RNA, translated into proteins that drive cellular functions.
Key Principles of SynBio
- Modularity: Standardized genetic parts, or “BioBricks,” such as promoters (gene switches), coding sequences, and terminators, enable plug-and-play designs. These are cataloged in repositories like the iGEM Registry (est. 2003).
- Chassis Organisms: Minimalist microbes (e.g., E. coli JCVI-syn3.0) or yeast serve as customizable platforms for hosting synthetic circuits.
- Design-Build-Test-Learn (DBTL) Cycles: Iterative workflows combine computational design, DNA synthesis, lab testing, and refinement to optimize systems.
- Orthogonality: Synthetic components (e.g., unnatural amino acids) operate independently of natural biology, enhancing safety and control.
Historical Milestones
SynBio’s trajectory reflects decades of innovation:
- 1972: Paul Berg’s recombinant DNA merges viral and bacterial genes, birthing genetic engineering.
- 2000: Tom Knight proposes BioBricks, standardizing genetic parts.
- 2003: iGEM launches, turning students into bioengineers.
- 2010: J. Craig Venter’s team creates JCVI-syn1.0, the first synthetic cell with a chemically synthesized genome.
- 2012: CRISPR-Cas9 emerges, enabling precise genome editing.
- 2020s: DNA synthesis costs plummet to ~$0.10 per base pair, democratizing access.
These advances set the stage for AI to amplify SynBio’s potential, tackling the field’s central challenge: biology’s combinatorial complexity.
AI as SynBio’s Catalyst: From Tinkering to Generative Design

Biological systems are dauntingly complex. A single protein’s sequence space (20^100 for a 100-amino-acid chain) dwarfs computational limits. AI, with its ability to parse massive datasets and model non-linear interactions, is the perfect partner. Machine learning (ML)—spanning deep neural networks, generative adversarial networks (GANs), and reinforcement learning—transforms SynBio in three ways:
- Generative Design: AI invents novel biomolecules (DNA, proteins, pathways) tailored to specific functions, bypassing nature’s constraints.
- Predictive Modeling: Physics-informed neural networks (PINNs) simulate molecular dynamics, predicting outcomes with >90% accuracy in silico.
- Automation: AI-driven biofoundries integrate robotics and ML to execute thousands of DBTL cycles daily.
Mathematically, AI reframes SynBio as an optimization problem. For example, protein design uses variational autoencoders (VAEs) to map sequences to functions, minimizing loss functions like root-mean-square deviation (RMSD) against target structures. Reinforcement learning further refines designs by rewarding functional outcomes, compressing timelines from years to days.
Breakthroughs at the AI-SynBio Frontier (2020–2025)
The past five years mark a “deluge” of AI-SynBio innovations, as noted in a 2025 Cell review. Here are pivotal advances:
1. Protein Engineering
- AlphaFold2 (2020): DeepMind’s AlphaFold solved protein folding, predicting structures with atomic precision. This unlocked de novo enzyme design, e.g., cellulases for biofuels with 2x higher yields.
- ESM3 (2023): EvolutionaryScale’s model simulated 500 million years of evolution, generating esmGFP—a fluorescent protein with 20% brighter output and enhanced photostability for medical imaging.
- 2025: AI-designed enzymes degrade PET plastics in hours, a 10x speedup over natural counterparts.
2. Genetic Circuits
- 2022: Diffusion models, inspired by image generation, crafted synthetic promoters with tunable expression in mammalian cells, enabling precise gene control (e.g., insulin production in pancreatic cells).
- 2025: A Cell study showcased AI-designed DNA regulators that activate genes in erythroid progenitors for anemia therapies, with 95% cell-type specificity.
3. CRISPR Optimization
- 2024: Generative AI produced orthogonal CRISPR-Cas systems, reducing off-target edits by 100x. This enables safer gene therapies for diseases like sickle cell anemia.
4. Lab Automation
- 2024: Genentech’s “lab-in-a-loop” used reinforcement learning to evolve antibodies, achieving 3–100x affinity improvements for cancer targets like EGFR.
- 2025: AI-orchestrated biofoundries (e.g., Ginkgo Bioworks) synthesize 10,000 genetic constructs daily, slashing drug development costs by 50%.
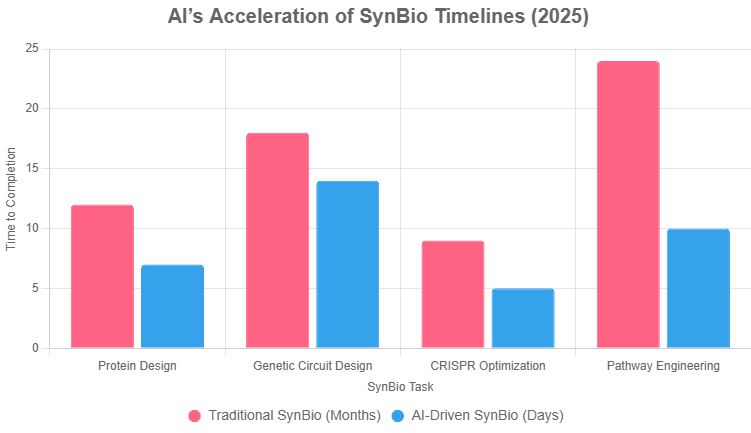
The chart below quantifies AI’s impact on SynBio timelines, comparing traditional vs. AI-driven DBTL cycles.
Economic Impacts: A Booming Bioeconomy
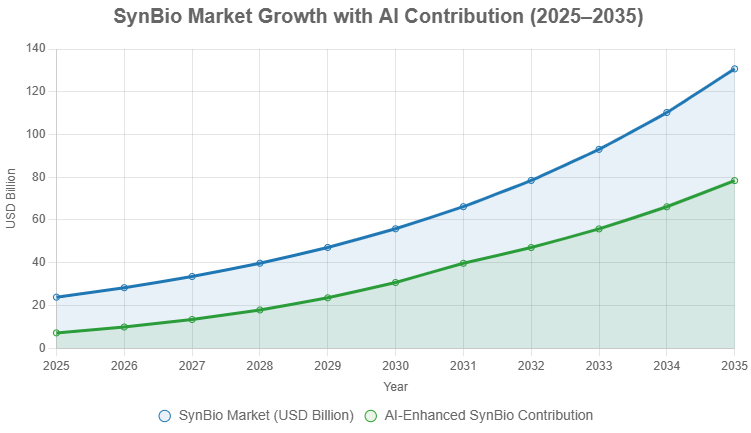
The AI-SynBio nexus is fueling a bioeconomic renaissance. The global SynBio market, valued at USD 23.88 billion in 2025, is projected to soar to USD 130.67 billion by 2035 (CAGR 18.53%), with therapeutics (45% share) and sustainable materials leading. AI’s role—optimizing designs and scaling production—drives this growth.
Market Drivers
- Therapeutics: AI-designed biologics (e.g., insulin, antibodies) dominate, with mRNA vaccines developed in days.
- Sustainability: Engineered microbes produce biofuels and bioplastics, reducing reliance on fossil fuels.
- Agriculture: AI-CRISPR crops boost yields by 20–30% in climate-stressed regions.
The chart below projects market growth, highlighting AI’s catalytic effect.
Patents tell a similar story: SynBio filings grew from ~1,000 in 2010 to >10,000 annually by 2023, with AI-driven designs comprising 30%. Publications surged, with the U.S. leading (20,306 papers, 33.6% global share, 2012–2023).
Real-World Applications: Rewriting Life’s Possibilities
AI-SynBio is already reshaping industries:
- Medicine: AI-optimized CAR-T cells target solid tumors with 80% efficacy in preclinical trials. Rapid mRNA vaccine design, as seen in 2023’s mpox response, takes 48 hours.
- Sustainability: AI-engineered algae capture CO₂ at 10x natural rates while yielding biofuels. Plastic-degrading enzymes achieve 90% PET breakdown in hours.
- Agriculture: AI-CRISPR crops enhance drought tolerance, boosting yields by 20–30%.
- Materials: Bacteria produce spider silk or living bone scaffolds, programmable via AI-designed circuits.
- Space Exploration: NASA explores AI-SynBio microbes for Martian resource production, synthesizing nutrients in extreme conditions.
Companies like Zymergen (chemicals), Asimov (circuits), and Amyris (biofuels) are scaling these solutions, with AI biofoundries producing 10x more constructs than manual labs.
Ethical and Societal Challenges
The AI-SynBio nexus amplifies both promise and peril:
- Biosecurity: AI could design novel pathogens, outpacing current screening. A 2025 Nature review warned of “generative biology” enabling biothreats. Mitigation includes AI-enforced kill switches and sequence filters.
- Equity: Tools remain concentrated in wealthy nations, risking a bioeconomic divide. Open-source platforms like iGEM help, but access gaps persist.
- Ethics: Designer organisms blur natural/artificial lines, raising questions about “playing God.” The WHO’s 2025 SynBio guidelines call for global oversight.
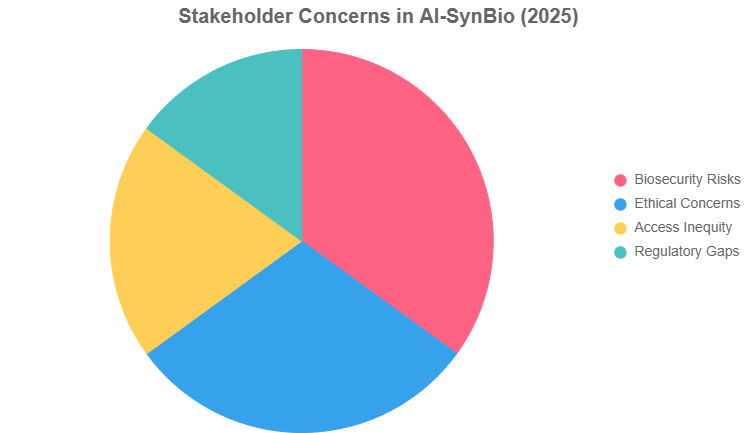
The chart below visualizes stakeholder concerns, drawn from 2025 global surveys.
The Future: A Programmable Biosphere
By 2030, AI-SynBio could make biology as editable as code. Potential futures include:
- Personalized Medicine: AI-tailored therapies for rare diseases, with 100x faster drug discovery.
- Planetary Engineering: Microbes terraforming degraded ecosystems or extraterrestrial environments.
- Bio-Computing: DNA-based circuits rivaling silicon chips in speed and storage.
Yet, as George Church notes, “We’re not playing God; we’re apprentices to nature’s complexity.” Success hinges on balancing innovation with governance. The WHO, NIH, and EU are drafting function-based regulations, but global coordination remains critical.
Conclusion: A Call to Shape the Biofuture
The AI-SynBio revolution is here, compressing decades of progress into years. From esmGFP’s glow to CO₂-munching algae, we’re witnessing life’s programmability unfold. But with great power comes great responsibility. Researchers, policymakers, and citizens must co-create a future where this technology serves all, not just a few.
Leave a comment